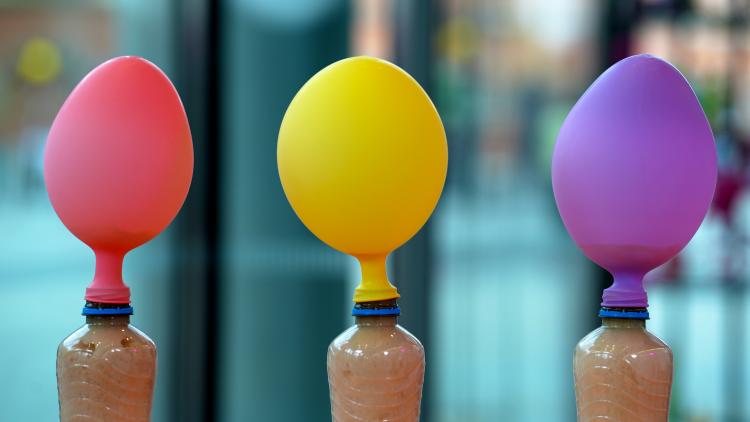Try some chemical reactions
This activity will take 30 minutes, is for ages 7 to 10 with supervision needed.
Create your own chemical reactions using bicarbonate of soda and sugar. See if they behave differently, despite looking the same to our eyes.
What you'll need
- Bicarbonate of soda
- A powder that looks similar to bicarbonate of soda, such as flour or icing sugar
- Vinegar
- A plastic drink bottle that's around 500ml
- A balloon or plastic bag. If using a plastic bag, make sure it doesn't have any holes in it.
- A hair tie or rubber band (if you're using a plastic bag)
- A spoon
What to do
- Pour vinegar into the plastic drinks bottle until it is around two to three centimetres full.
- Make a funnel with a piece of paper and use it to put two spoons of bicarbonate of soda into the balloon or plastic bag.
- Fasten the balloon or bag around the rim of the drinks bottle (or the plastic bag using the hair tie) being careful to not let any of the powder fall into the vinegar.
- Upend the balloon or plastic bag quickly to let all of the powder fall into the vinegar, making sure you keep it sealed around the rim of the bottle.
- Watch the balloon start to inflate!
- Try this experiment again with another powder that looks similar to bicarbonate of soda such as flour or icing sugar. Does the balloon still inflate?
The science
Chemical reactions happen when chemicals, (e.g. the acid vinegar and the base sodium bicarbonate) are changed into something new. In this case the reaction makes a gas, carbon dioxide, which blows up the balloon.
When you substitute the sodium bicarbonate with something that looks similar, such as icing sugar, the reaction doesn’t work anymore because the icing sugar doesn’t have the same chemical properties as the sodium bicarbonate.
Science at the Crick
Acids can also be found in living things. Chemical reactions involving amino acids are needed for cell growth.
Scientists at the Crick are investigating how the bacterium that causes the disease tuberculosis (TB) can be killed. We've found that tuberculosis can be treated by giving people a drug that looks a lot like the amino acid the bacteria need to grow. Just like in our experiment, even though the drug looks similar to the amino acid, it's different, and the chemical reaction needed for cell growth doesn’t happen. This means the bacteria can’t grow properly and the disease doesn't develop.